Conservation Status

Juniperus excelsa
M.Bieb. (1798)
Common names
Greek juniper.
Taxonomic notes
Synonymy: see POWO. Older treatments of this species commonly include J. polycarpos and J. seravschanica as synonyms or infraspecific taxa, but they are now recognized at species rank due to the substantial morphological, molecular, and ecological differences between the taxa, as well as their disjunct ranges (Adams 2004, Adams et al. 2008, Adams 2014, Adams et al. 2014). This is one of 8 species in a widely-distributed clade (Macaronesia to Japan) within Juniperus section Sabina. It is sister to J. polycarpos and J. seravschanica. Both molecular and morphological studies indicate there is not a great deal of intraspecies variation in J. excelsa (Mazur et al. 2004; Douaihy et al. 2011, 2012).
Description
Monoecious or dioecious evergreen trees (sometimes shrubs or prostrate shrubs) to 25 m tall and 250 cm dbh, usually with a single trunk; crown broadly pyramidal in young trees, with age becoming broad, irregular, or shrub-like to prostrate in alpine locations. Bark first smooth, soon with papery flakes, reddish-brown, later fibrous, becoming longitudinally furrowed and peeling in long strips; purplish- to reddish-brown. Twigs numerous, arrayed in dorsiventral sprays in young trees, or more irregular and very dense, esp. in dry environments; ultimate twigs covered with leaves, round, diameter 0.8-1 mm. Juvenile leaves only on young plants; ternate, acicular, c. 5-6 × 1 mm, widest at base, keeled, pungent; mature leaves scale-like, decussate, imbricate, appressed or free at the mostly incurved apex, decurrent at base, ovate-rhombic on ultimate branchlets, (ob)lanceolate-acute on older shoots, 1-1.5 mm long, with entire margins; glands ovate to linear; stomata on juvenile leaves epistomatic, on mature leaves amphistomatic, mostly in 2 inconspicuous lines tapering from base to apex, light green or yellowish-green; both juvenile and mature leaves have a single median resin cavitv. Pollen cones numerous, solitary and terminal or subterminal on ultimate branchlets, 3-4 × 2-3 mm, greenish maturing yellowish; microsporophylls 8-10, peltate with rounded, thin margins, each bearing 3-4 pollen sacs. Seed cones numerous, mostly solitary and axillary, subterminal on ultimate branchlets, sessile; young cones surrounded by green leaves or bracts, 2-3 mm diameter, purplish-green to blue; mature cones globose, 8 mm diameter, slightly glaucous, dark purplish-brown at maturity; seed scales 4(-6), decussate, entirely fused with bracts and each other, the two largest meeting at the distal pole of the cone, 4-9 mm long, surface smooth, waxy, with a ridge terminating in a small umbo (0.5-0.6 mm), interior resinous, becoming woody, yellowish. Seeds 4-6 per cone, angular, broadest at base, ovoid but flattened or curved, 4-6 × 3-4 mm, yellowish to reddish-brown (Farjon 1992, Adams 2014). See García Esteban et al. (2004) for a detailed characterization of the wood anatomy.
Distribution and Ecology
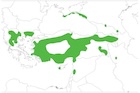
Albania, North Macedonia, S Bulgaria, Greece, Turkey, Cyprus, Syria, Lebanon, Georgia, Russia, Ukraine, Azerbaijan, Armenia (see map at right). Its abundance varied widely through this large range, but it is generally a species of the mountains. In the southern and central Balkans and the mountains of Cyprus it occurs in rare and fragmented woodlands. It is regionally widespread and continuously distributed along the Taurus Mountains of southern Anatolia, but occurrences are severely fragmented on the Anatolian plateau and in the mountains of Syria and Lebanon. It grows mainly on stony, rocky calcareous or non-calcareous slopes. It is most frequently found in cold Mediterranean zones within in lower sub-humid climates (annual precipitation 500-1000 mm), at elevations of 1000-1300 m in the Anatolian forests, and at 1600-1800 m on Mount Lebanon. Its lowest-elevation occurrences are at c. 100 m in Crimea, but it can reach maximum elevations of 2100 m in Greece and some individuals can be found at elevations as high as 2700–2800 m in the Taurus and on Mount Lebanon. It can tolerate severe drought and cold, and can grow on shallow, degraded soils. It is the dominant tree species found at the tree line in the eastern Mediterranean region with very sparse pure vestigial stands. It may form pure, open forests, grow mixed with J. foetidissima or with other conifers such as Cedrus libani, Cupressus sempervirens and Pinus spp., or it may be part of oak-scrub communities in secondary vegetation, but not in Mediterranean maquis (Farjon 1992; Douaihy et al. 2011, 2012 and sources therein). Like other junipers, it is wind-pollinated and the seeds are dispersed by birds and small mammals (Douaihy et al. 2011).
Hardy to Zone 6 (cold hardiness limit between -23.2°C and -17.8°C) (Bannister and Neuner 2001).
The IUCN has assessed this species (as J. excelsa subsp. excelsa) as of "Least Concern" for conservation due to its abundance within a wide range, and the absence of indicators for overall decline.
Remarkable Specimens
The oldest known specimen, 915 years, is documented in a tree-ring chronology covering the period 1017-2006 (fully crossdated), collected near Elmali, Turkey by Ramzi Touchan and colleagues (doi.org/10.25921/18v7-xv38). The 915-year lifespan was shown in one sample that ended in 1990 and thus was presumably dead. The chronology was used in at least one paleoclimate study (Touchan et al. 2005).
Ethnobotany
The wood is very durable and of good quality (Vidakovic 1991).
Observations
"Macedonia is the northwest border of its distribution. There it occurs also in almost pure stands around the Vardar River, Pcinja, Anska Reka, Demir-Kapija, Crna Reka and Lake Prespansko, between 100 and 800 m elevation, on lime and silicate mother rocks" (Vidakovic 1991).
Remarks
The epithet excelsa means "high", perhaps referring to how tall this species gets, compared to other European junipers, though it may also refer to its occurrence in mountains, even at the timberline.
J. excelsa is thought to have had Pleistocene glacial refugia in the Taurus–Ammanus and Cyprus–Lebanon mountains; also, macrofossils dating to the last glacial maximum have been found in the eastern Balkan peninsula (Douaihy et al. 2011, 2012 and sources therein).
Citations
Adams, Robert P. 2004. Juniperus deltoides, a new species, and nomenclatural notes on Juniperus polycarpos and J. turcomanica (Cupressaceae). Phytologia 86(2):49–53.
Adams, Robert P. 2014. Junipers of the World: The Genus Juniperus. Fourth edition. Trafford Publishing. Brief versions of the descriptions are available online at Adam's website, www.juniperus.org.
Adams, Robert P., Julie A. Morris, and Andrea E. Schwarzbach. 2008. Taxonomic study of Juniperus excelsa and J. polycarpos using SNPs from nrDNA and Cp trnC-trnD plus essential oils and RAPD data. Phytologia 90(2):216–33.
Adams, Robert P., Boucha Douaihy, Magda Dou Dagher-Kharrat, Andrea E. Schwarzbach, and Vahid Farzaliyev. 2014. Geographic variation in nrDNA and four cpDNA regions of Juniperus excelsa and J. polycarpos from Greece, Turkey, Lebanon and Azerbaijan. Phytologia 96(2):89–95.
Bieberstein, Maschall von. 1798. Tabl. Prov. Mer Casp.: 120.
Douaihy, Bouchra, Giovanni G. Vendramin, Adam Boratyński, Nathalie Machon, and Magda Bou Dagher-Kharrat. 2011. High genetic diversity with moderate differentiation in Juniperus excelsa from Lebanon and the eastern Mediterranean region. AoB PLANTS https://doi.org/10.1093/aobpla/plr003.
Douaihy, Bouchra, Karolina Sobierajska, Anna Katarzyna Jasińska, Krystyna Boratyńska, Tolga Ok, Angel Romo, Nathalie Machon, Yakiv Didukh, Magda Bou Dagher-Kharrat, and Adam Boratyński. 2012. Morphological versus molecular markers to describe variability in Juniperus excelsa subsp. excelsa (Cupressaceae).” AoB PLANTS https://doi.org/10.1093/aobpla/pls013.
Mazur, Małgorzata, Krystyna Boratyńska, Katarzyna Marcysiak, Yakov Didukh, Angel Romo, Piotr Kosiński, and Adam Boratyński. 2004. Low level of inter-populational differentiation in Juniperus excelsa M. Bieb. (Cupressaceae). Dendrobiology 52:39–46.
Touchan, R., E. Xoplaki, G. Funkhouser, J. Luterbacher, M.K. Hughes, N. Erkan, Ü. Akkemik, and J. Stephan. 2005.
Reconstructions of spring/summer precipitation for the eastern Mediterranean from tree-ring widths and its connection to large-scale atmospheric circulation. Climate Dynamics 25:75-98.
See also
Elwes and Henry 1906-1913 at the Biodiversity Heritage Library. This series of volumes, privately printed, provides some of the most engaging descriptions of conifers ever published. Although they only treat species cultivated in the U.K. and Ireland, and the taxonomy is a bit dated, still these accounts are thorough, treating such topics as species description, range, varieties, exceptionally old or tall specimens, remarkable trees, and cultivation. Despite being over a century old, they are generally accurate, and are illustrated with some remarkable photographs and lithographs.
Farjon (2005) provides a detailed account, with illustrations.
Adams, Robert P. 1990. Juniperus procera of East Africa: volatile leaf oil composition and putative relationship to J. excelsa. Biochemical Systematics and Ecology 18(4):207-210.
Adams, Robert P. 1990. The chemical composition of leaf oils of Juniperus excelsa M.-Bieb. Journal of Essential Oil Research 2:45-48.
Adams, Robert P., R. K. Thappa, S. G. Agarwal, B. K. Kapahi, and Y. K. Sarin. 1992. The volatile leaf oils of Juniperus semiglobosa Regel from India compared with J. excelsa M.-Bieb. from Greece. Journal of Essential Oil Research 4:143–149.
Adams, Robert P., Tigst Demeke, and H. A. Abulfatih. 1993. RAPD DNA fingerprints and terpenoids: clues to past migrations of Juniperus in Arabia and East Africa. Theoretical and Applied Genetics 87:22–26.
Adams, Robert P., Alexander N. Tashev, K. Husnu Can Baser, and A. K. Christou. 2013. Geographic variation in the volatile leaf oils of Juniperus excelsa M.-Bieb. Phytologia 95(4):279–285.
Adams, Robert P., Boucha Douaihy, Magda Dou Dagher-Kharrat, Vahid Farzaliyev, Alexander N. Tashev, K. Husnu Can Baser, and A. K. Christou. 2014. Geographic variation in the volatile leaf oils of Juniperus excelsa and J. polycarpos. Phytologia 96(2):96–106.
Salehi Shanjani, Parvin, Mehdi Mirza, Mohsen Calagari, and Robert P. Adams. 2010. Effects drying and harvest season on the essential oil composition from foliage and berries of Juniperus excelsa. Industrial Crops and Products 32(2):83–87. https://doi.org/10.1016/j.indcrop.2010.03.003.